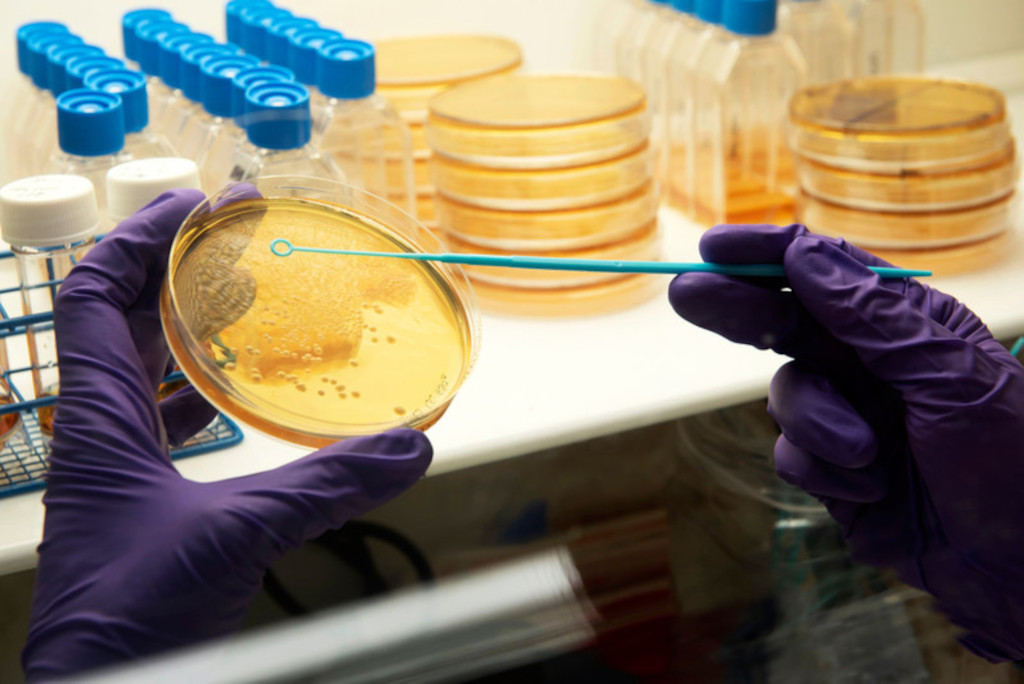
Technologies available for licensing
Detecting insecticide levels on bed nets
Bed nets coated with insecticides against mosquitos are essential in reducing the transmission and spread of malaria. They are replaced frequently to maintain protection against mosquitos. The technology involves a fast and low cost in situ colorimetric test detecting insecticide levels in bed nets, avoiding the unnecessary replacement of bed nets particularly in areas affected by malaria.
Artemisinin drug assay
Artemisinin-based combination therapies (ACT) are the first-line malaria treatment recommended by the WHO. Drug quality in many areas of the world however represents a significant concern, with many drugs in the supply chain containing inadequate levels of active pharmaceutical or no active pharmaceutical at all. The technology is a simple, robust, rapid and portable field test with a two-step protocol to provide an assessment of artemisinin drug quality.
Dust mite lure
Dust mites are common to households throughout the world and are the primary indoor allergen source and trigger of allergic asthma, dermatitis and rhinitis. They are difficult to remove from households because they cling deep within the fibres of household furniture. This technology involves a low-cost composition of chemicals that lure dust mites to the surface of furniture so that they can be removed with traditional cleaning methods.
Tularaemia Vaccine
F. tularensis is one of the most infectious pathogens known to man and is endemic to the northern hemisphere. Due to its extremely low infectious dose, ease of spread by aerosol and high virulence, F.tularensis is a potential bio-weapon. A low-cost, versatile protein-glyconjugate vaccine for F.tularensis was developed using a novel protein-glycosylation coupling technology. Vaccine was shown to have 100% efficacy in infection models.
Pneumococcal Vaccine
S. pneumoniae is the causative pathogen for pneumococcal infections that include pneumonia, septicemia, and meningitis, with a mortality rate of up to 35% for the more serious infections. A low-cost, versatile pneumococcal vaccine has been developed using a novel protein-glycosylation coupling technology. This vaccine can be tailored to target different serotypes of S. pneumoniae.
African Horse Sickness Vaccine
African Horse Sickness (AHS) is an arthropod-borne viral disease affecting horses with a mortality rate of over 75% upon infection. Researchers at LSHTM have developed a novel form of AHS vaccine using Entry Competent Replication Abortive (ECRA) viruses. This novel form of vaccine technology provides great advantage over commercially available vaccines in that they are safer, cheaper to transport and provide complete serotype coverage.
Blue Tongue Sickness Vaccine
Blue Tongue Virus (BTV) affects sheep, cattle, deer, goats and camelids with mortality rate of 30-70% upon infection. A replication-deficient form of the virus (ECRA) has been developed as a vaccine which provide great advancement in the treatment and control of BTV. These BTV ECRA vaccines are cheaper to transport, can be applied as cocktail to target different serotypes and are safer than currently available vaccines on the market.
Tuberculosis therapeutics and screening assay
Tuberculosis (TB) is one of the leading infectious causes of death worldwide. Each year the disease is becoming more challenging to treat due to drug resistance. This technology offers a new therapeutic opportunity to target Mycobacterium tuberculosis complex (MTBC) species whereby protein kinase inhibitors can directly inhibit MTBC species growth. Additionally, an assay has been developed for investigating the anti-MTBC abilities of the protein kinase inhibitors.
Further advice
Find a consultant
There are more than 3,000 staff at LSHTM at the forefront of knowledge and expertise in global health and infectious disease. LSHTM staff are experienced in providing expert advice to governments and NGOs informing policies and best practice. LSHTM can also provide services in testing and analysis. If you are looking for a consultant or would like to find out more about consultancy services at LSHTM then please email info@chariotinnovations.co.uk

Opportunities to invest & Partnerships
Chariot Innovations has the ambition to drive the commercialisation of research originating from LSHTM. Providing sufficient capital at early stages of translational activity is a key catalyst for successful commercial development. There are many opportunities to invest in early-stage projects with impact, and we are always open to partner with VC firms, angel investors and other enterprises interested in funding innovation and spin-outs. Please email info@chariotinnovations.co.uk to learn more and start a discussion.